
Doping turns pure silicon into a semiconductor by adding or removing a very, very small number of electrons, thereby making it neither an insulator nor a conductor, but a semiconductor with limited charge conduction.

Doping refers to a process by which impurities are introduced into ultra-pure silicon, thereby changing its electrical properties and turning it into a semiconductor. This crystalline structure is turned into a semiconductor when it is doped.
NUMBER OF VALENCE ELECTRONS IN SI ELEMENT FREE
However, this silicon lattice is essentially an insulator, as there are no free electrons for any charge movement, and is therefore not a semiconductor. Figure 15: An example of a silicon lattice

(see Figure 15) Silicon is perfect for making this lattice structure because its four valence electrons allow it to perfectly bond to four of its silicon neighbors. This structure is called a silicon lattice. Like carbon, silicon can make a diamond-like crystal. But semiconductors are not made out of silicates, or silanes, or silicones they are made out of pure silicon, that is, essentially pure silicon crystal. Semiconductors can be made out of a variety of materials, but the majority of semiconductors are made out of silicon. They are used to rectify, amplify, and switch electrical signals and are thus integral components of modern day electronics. Semiconductors are able to manipulate electric current. Semiconductors lie somewhere in between these two classes, giving them a very useful property. Semiconductors are unique materials that have neither the electrical conductivity of a conductor nor that of an insulator. Among other things, silanes are used as water repellents and sealants. Silanes have a variety of industrial and medical uses. (see Figure 12) Figure 12: The organosilane, dichlorodimethylsilane Silanes also have a tendency to swap out their hydrogens for other elements and become organosilanes. Silanes are particularly prone to decomposition via reaction with oxygen. In fact, silanes are rather prone to decomposition. Hexasilane is the largest possible silane because Si-Si bonds are not particularly strong. Figure 11: The largest silane, hexasilane The largest silane has a maximum of six silicon atoms (see Figure 11). But there is a very quick end to this trend. Like hydrocarbons, silanes progressively grow in size as additional silicon atoms are added. But silicon does not play an integral role in our day to day biology. With the same valence configuration, and thus the same chemical versatility, silicon could conceivably play a role of similar organic importance. Carbon-hydrogen compounds form the backbone of the living world with seemingly endless chains of hydrocarbons. Silicon is an integral component in minerals, just as Carbon is an essential component of organic compounds. The tetrahedral SiO 4 4 - complex (see Figure 3), the core unit of silicates, can bind together in a variety of ways, creating a wide array of minerals. Figure 3: This is a representation of the tetrahedral silica complex The empirical form of silica is SiO 2 because, with respect to the net average of the silicate, each silicon atom has two oxygen atoms. This leads to silicates linking together in -Si-O-Si-O- networks called silicates. silicon dioxide, takes on this molecular form, instead of carbon dioxide's characteristic shape, because silicon's 3p orbitals make it more energetically favorable to create four single bonds with each oxygen rather than make two double bonds with each oxygen atom.
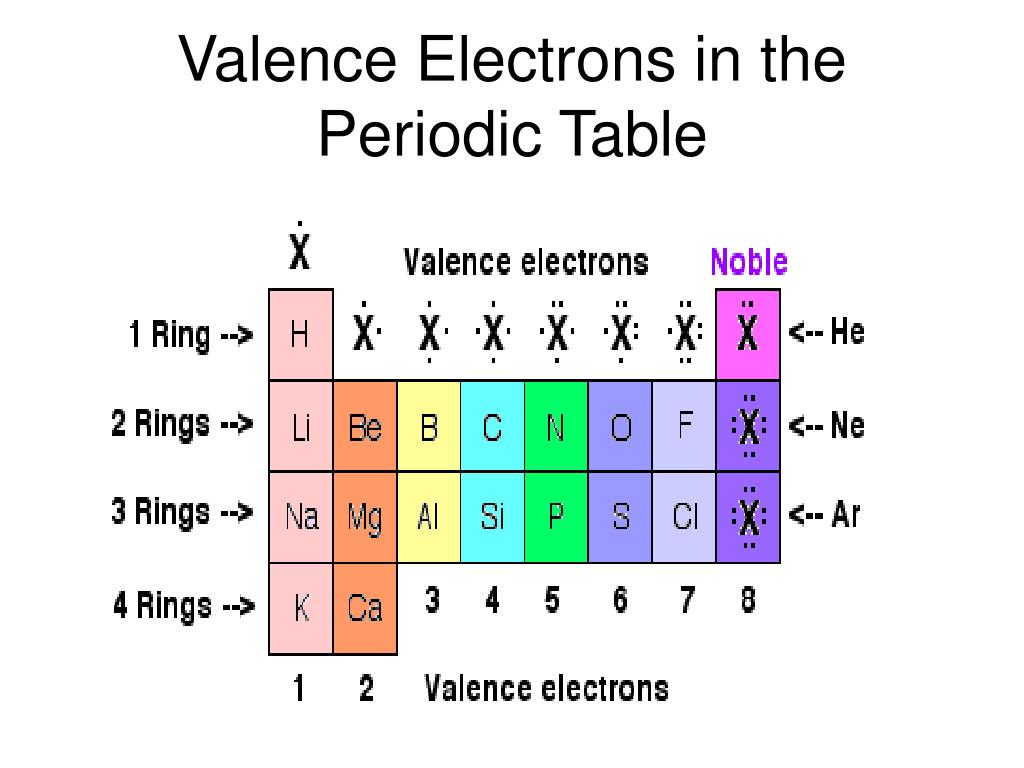
Figure 2: The net charge of silica is minus 4 Silica is composed of one silicon atom with four single bonds to four oxygen molecules (Figure 2). Silica is not a silicon atom with two double bonds to two oxygen atoms. Silica is the one stable oxide of silicon, and has the empirical formula SiO 2. Silicon is most commonly found in silicate compounds.


 0 kommentar(er)
0 kommentar(er)
